Clinical evidence
pHyph is an approved medical device which confirms its the efficacy and safety.In the ambitious clinical program in bacterial vaginosis, pHyph has shown to treat bacterial vaginosis and promote a healthy microbiome.
Bacterial vaginosis
Gedea has run an ambitious clinical program for pHyph in bacterial vaginosis. Three clinical studies have demonstrated the safety and effective treatment of bacterial vaginosis. The most recent clinical trial, NEFERTITI-2 shows gignificantly higher cure rate for pHyph than for the controlgroup.
The recently finalized NEFERTITI-2 study for treatment and prevention of BV with pHyph with 61 patients showed statistically significant results compared to the control group, which was the study’s primary endpoint. The purpose was to investigate the cure rate upon treatment with pHyph compared to an untreated control group (1:1 ratio). Cured patients continued with a twice weekly pHyph dosing to prevent recurrence for up to two months. The maintenance treatment was shown to maintain the initial treatment improvement of the vaginal microbiome. The study was conducted at five Swedish sites. The study was designed to include 92 patients but as the primary endpoint was statistically significant already at the interim analysis at 61 patients, the study was concluded ahead of plan. Results showed that 63% of patients were completely free of the fishy-smell at day 7 and 77% of patients experienced alleviation after treatment.

N=61
Active: Control 1:1

63% free of fishy smell day 7
77% alleviation of symptoms

Secondary vaginal
fungal infection: 0%

Recurrence day 35: 0 %
Follow up: 2 months
The NEFERTITI (CL3) study included 152 patients with BV and showed a rapid onset of symptom reduction, where 70% of patients were completely free of the fishy-smell and 90% of patients experienced alleviation after treatment. The cure rate was in line with antibiotic products on the market. Furthermore, pHyph treatment showed a considerably lower recurrence rate compared to placebo, both at day 35 (pHyph: 13.9%, Placebo: 27%) and throughout the four-month follow-up period. The placebo arm was impacted by an unexpected effect of the placebo tablet filling material which promoted growth of lactobacilli, subsequently causing a pH reduction, and relieving the BV symptoms, a finding which led to a new patent filing. There were no safety concerns. Further analysis on occurrence of vaginal dysbiosis by microbiome analysis are ongoing.

N=152
Active: Placebo 4:1

Recurrence day 35: 13,9%
Follow up: 128 days (4 months)

Secondary vaginal
fungal infection: 0%

70% free of fishy smell day 7
90% alleviation of symptoms
The CL2-study included 24 patients with BV and showed 82% cure rate in a week, whereas only 5.6% of patients had a recurrence at day 35. No patients experienced secondary candida infections and there were no safety concerns. The results have been published in the European Journal of Obstetrics & Gynecology and Reproductive Biology.

N=24
Active treatment

Recurrence day 35: 5,6%
Follow up period: 35 days

Secondary vaginal
fungal infection: 0%

82% free of all
symptoms day 7
The vaginal microbiome
There are always microorganisms, as bacteria and fungi, in and on our bodies. It is called the microbiome and is necessary for our health. In the healthy vaginal microbiome there is a balance between different bacteria and fungi, depending on factors as pH-level, medication, hormones and other factors.
A healthy vaginal microbiome is dominated by Lactobacillus ssp. which produce lactic acid, hydrogen peroxide and various antimicrobial compounds. An imbalance in the normal bacterial flora of the vagina is called dysbiosis. This condition is characterized by a reduction in beneficial bacteria, such as Lactobacillus species, and an overgrowth of potentially harmful bacteria. Dysbiosis is associated with adverse gynecologic and obstetric outcomes, such as sexually transmitted infections, pelvic inflammatory disease, and preterm birth.
Microbiome analysis
There are always microorganisms, as bacteria and fungi, in and on our bodies.
The microbiome is of key importance to treat and prevent bacterial vaginosis.

Antibiotics and the microbiome

Traditional antibiotic treatment for BV negatively affects the vaginal microbiome, reducing resistance to new infections. As a result, 23-46% of those treated with antibiotics for BV experience a recurrence of symptoms within 30 days. pHyph helps to build and strengthen the natural microbiome, treating the infection and providing protection against new infections. Thus, the risk of recurrent infections is much lower with pHyph compared to antibiotics.
Microbiome research
Gedea runs an ambitious microbiome program together with Karolinska Institutet in women’s reproductive health, to describe the vaginal microbiome and it’s relation to health, fertility and pregnancy.
Ina Schuppe Koistinen, Ph.D. Associate professor, is supervisor to our Industrial Doctoral Student Emilia Lahtinen, Ph.D Student.

Pipeline
In the pipeline, pHyph is developed for vulvovaginal candidiasis (vaginal fungal infections) and for prevention of pre-term birth.
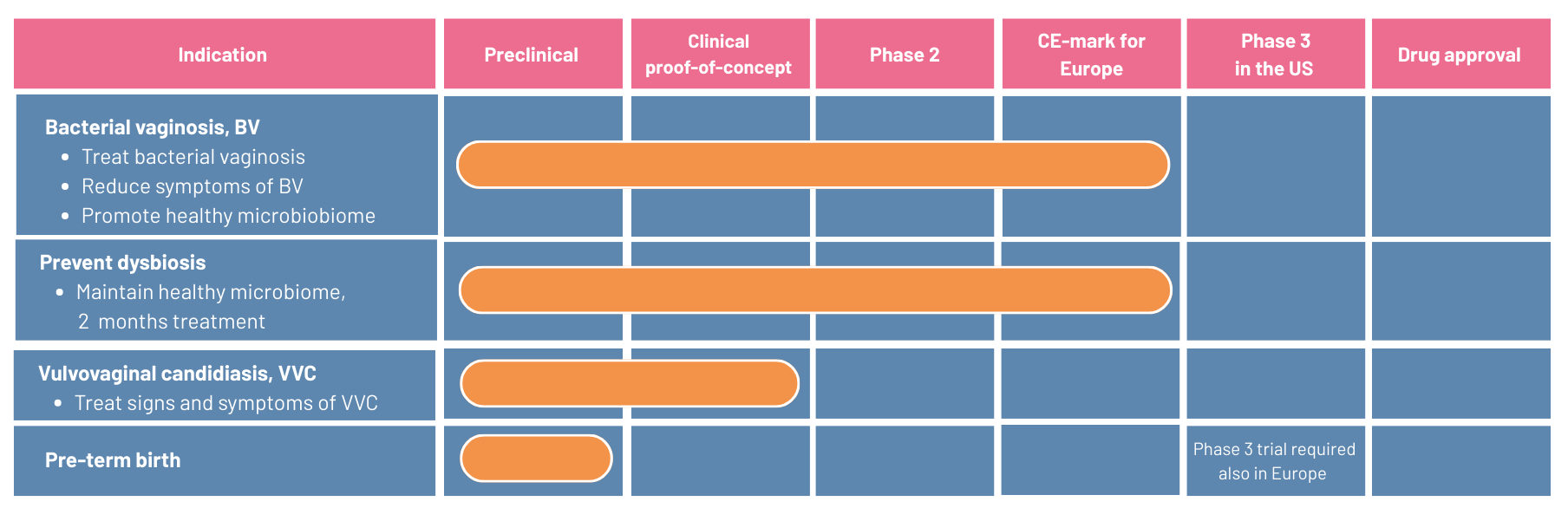
Vulvovaginal candidiasis
pHyph has established proof-of-concept in treatment of vulvovaginal candidiasis (VVC) in a recently finalized phase 2-trial (EpHect, CL4).
Many women perceive their symptoms as a vaginal yeast infection when, in fact, they are suffering from bacterial vaginosis, leading them to use antifungal treatments for their symptoms. This often results in repeated ineffective treatments and prolonged discomfort. An effective, over-the-counter treatment that works against both bacterial vaginosis and vaginal yeast infection would meet a significant need in the self-care of vaginal infections

Clinical results for vulvovaginal candidiasis
The EpHect (CL4) Proof-of-concept study for VVC
The EpHect study was a single armed clinical trial having enrolled 26 patients at four sites in Sweden. The primary endpoint was clinical cure rate. The patients were diagnosed with vulvovaginal candidiasis (VVC) at the initial clinical visit and treated at home with one vaginal tablet a day for six consecutive days, and clinical cure was assessed on day 7-14.
Top-line Results EpHect study
- 92% of the patients had a reduction in composite vulvovaginal signs and symptoms on day 7-14
- 62% of patients (17/26) were clinically cured day 7-14
- 88% of patients (22/25) thought pHyph was easy to use
- The product was well tolerated

N=26
Active treatment

92% alleviation of symptoms at day 7
62% clinical cure at day 7-14

Pre-term birth
Bacterial vaginosis, or the loss of Lactobacillus ssp. and increase in the concentration of anaerobic microbes, is a common disorder of the vaginal microbiota among women of reproductive age and associated with increased risk of preterm birth. Treatment with pHyph restores the microbiome
It is well-known that bacterial vaginosis and other imbalances in the vaginal microbiome during pregnancy are associated with an increased risk of preterm birth. If a pregnant woman’s microbiome is dominated by beneficial lactobacilli, she has a higher likelihood of giving birth full-term. Bacterial vaginosis, or the loss of Lactobacillus ssp. and increase in the concentration of anaerobic microbes, is a common disorder of the vaginal microbiota among women of reproductive age and associated
Next step
Further investigations of the product’s effect in preventing pre-term birth is a partnership opportunity.
Further development
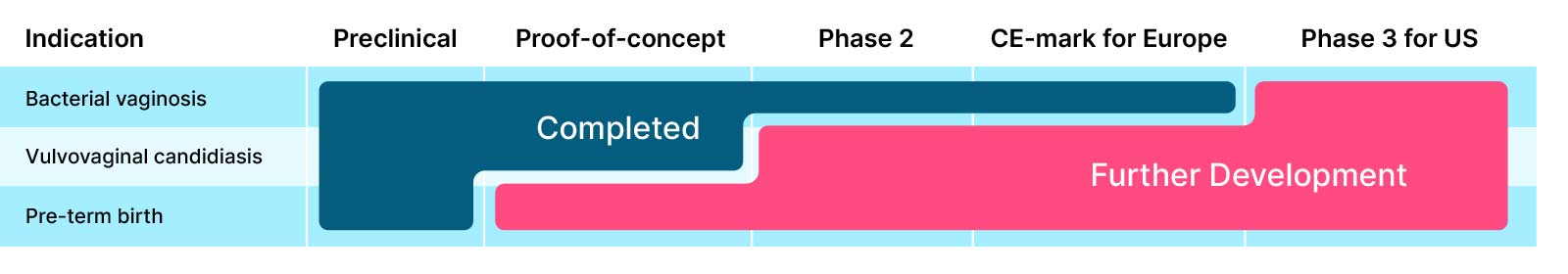
Bacterial vaginosis:
Phase-3 study for the US
pHyph is estimated to reach EU approval for treatment and prevention of bacterial vaginosis in 2025 and then be ready for phase 3 trials for US approval as an Rx-treatment. Gedea aims to close partnerships with larger pharma companies for sales and marketing as well as development for regulatory approvals worldwide of the product
Vulvovaginal candidiasis:
CE-mark for Europe and phase 3 study for the US
pHyph has established proof-of-concept in treatment of vulvovaginal candidiasis (VVC) in a recently finalized phase 2-trial (EpHect, CL4). It is an opportunity for a partner to investigate the possibility of a product with dual effect for treatment of both bacterial vaginosis and vulvovaginal candidiasis.
Pre-term birth
pHyph improves the vaginal microbiome and healthy microbiome is associated with full-term pregnancy. Further investigations of the product’s effect in preventing pre-term birth is a partnership opportunity.
Technical and biological data
Commercial scale production
Gedea has initiated the process for commercial scale manufacturing of pHyph. The tech transfer is ongoing and commercial production capacity is planned to be ready in time for the European product approval.
Technical data
The active substance in pHyph is a naturally occurring substance in our environment and is well documented, has GRAS status* and is used as an approved food additive, thus the product has a high safety profile. Gedea develops the first medical products where these substances are used as active ingredients, protected by our patent applications.
*Generally Recognized As Safe (GRAS) by the US Food and Drug Administration (FDA).

